2020
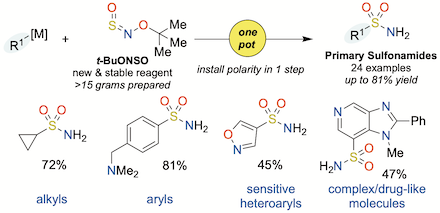
"Primary sulfonamide synthesis using the sulfinylamine reagent N-sulfinyl-O-(tert-butyl)hydroxylamine, t-BuONSO", Thomas Q. Davies, Michael J. Tilby, David Skolc, Adrian Hall, and Michael C. Willis, Org. Lett. 2020, 22, 9495–9499. (doi: 10.1021/acs.orglett.0c03505)
Highlighted on/in ChemistryViews, SynFacts, and the Organic Chemistry Portal.
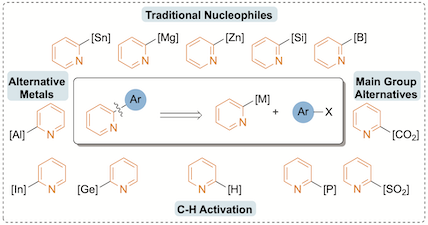
"The 2-pyridyl problem: Challenging nucleophiles in cross-coupling arylations", Xinlan A. F. Cook, Antoine de Gombert, Janette McKnight, Loïc R. E. Pantaine and Michael C. Willis, Angew. Chem. Int. Ed. 2021, 60, 11068–11091. (doi: 10.1002/anie.202010631)
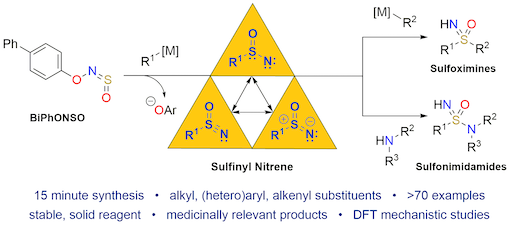
"Harnessing sulfinyl nitrenes: A unified one-pot synthesis of sulfoximines and sulfonimidamides", Thomas Q. Davies, Michael J. Tilby, Jack Ren, Nicholas A. Parker, David Skolc, Adrian Hall, Fernanda Duarte,and Michael C. Willis, J. Am. Chem. Soc. 2020, 142, 15445−15453. (doi: 10.1021/jacs.0c06986)
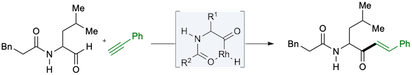
“alpha-Amidoaldehydes as substrates in rhodium-catalyzed intermolecular alkyne hydroacylation: The synthesis of alpha-amidoketones”, Ritashree Pal, Sean C. O'Brien and Michael C. Willis, Chem. Eur. J. 2020, 26, 11710-11714. (doi: 10.1002/chem.202002478).
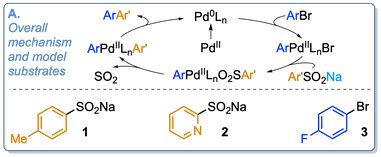
“Mechanism of the month: Palladium-catalyzed desulfinative cross-couplings”, Antoine de Gombert and Michael C. Willis, Trends in Chemistry 2020, 2, 865-866. (doi: 10.1016/j.trechm.2020.04.004)
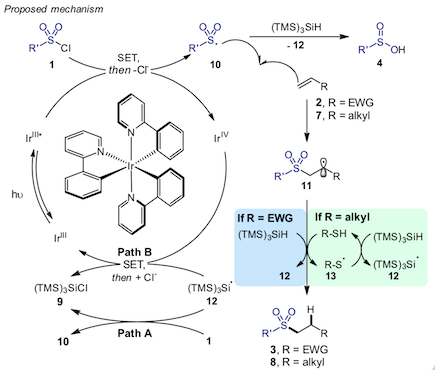
"Hydrosulfonylation of alkenes with sulfonyl chlorides under visible light activation”, Sandrine Monique Hell, Claudio Flavio Meyer, Antonio Misale, Jeroen B. I. Sap, Kirsten K. Christensen, Michael C. Willis, Andrés A. Trabanco, and Veronique Gouverneur,* Angew. Chem. Int. Ed. 2020, 59, 11620-11626. (doi: 10.1002/anie.202004070)
“Sulfinamide Synthesis using Organometallic reagents, DABSO and Amines”, Pui Kin Tony Lo, Gwyndaf A. Oliver, Michael C. Willis, J. Org. Chem. 2020, 85. 5753-5760. (doi: 10.1021/acs.joc.0c00334)
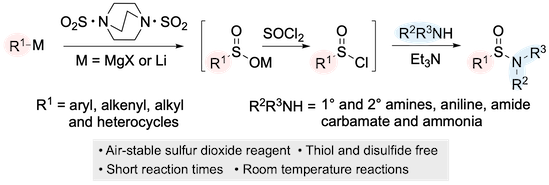
“Mechanistic studies of the palladium-catalyzed desulfinative cross-coupling of aryl bromides and (hetero)aryl sulfinate salts “, Antoine de Gombert, Alasdair I. McKay, Christopher Davis, Katherine M. Wheelhouse, and Michael C. Willis, J. Am. Chem. Soc. 2020, 142, 3564-3576. (doi: 10.1021/jacs.9b13260).
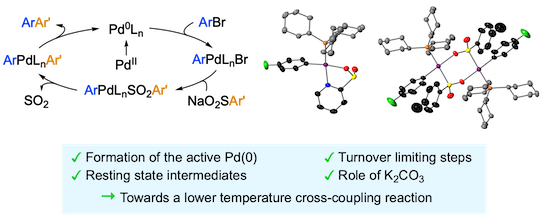
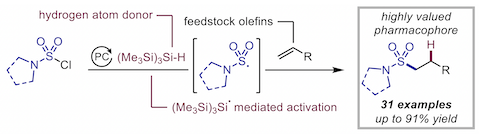
“Silyl radical-mediated activation of sulfamoyl chlorides enables direct access to aliphatic sulfonamides from alkenes”, Sandrine M. Hell, Claudio F. Meyer, Gabriele Laudadio, Antonio Misale, Michael C. Willis, Timothy Noël, Andrés A. Trabanco, and Véronique Gouverneur,* J. Am. Chem. Soc. 2020, 142, 720−725. (doi: 10.1021/jacs.9b13071)
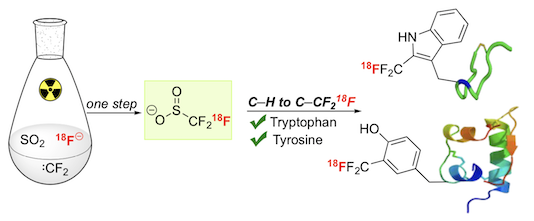
“18F-Trifluoromethanesulfinate enables direct C–H 18F-trifluoromethylation of native aromatic residues in peptides”, Choon Wee Kee, Osman Tack, Florian Guibbal, Patrick G. Isenegger, Thomas C. Wilson, Mateusz Imiołek, Stefan Verhoog, Michael Tilby, Giulia Boscutti, Sharon Ashworth, Juliette Chupin, Roxana Kashani, Adeline W. J. Poh, Jane K. Sosabowski, Sven Macholl, Christophe Plisson, Bart Cornelissen, Michael C. Willis, Jan Passchier, Benjamin G. Davis, Véronique Gouverneur,* J. Am. Chem. Soc. 2020, 142, 1180−1185. (doi: 10.1021/jacs.9b11709)
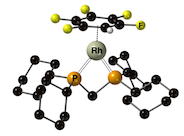
“Synthesis of highly fluorinated arene complexes of [Rh(chelating phosphine) cations, and their use in synthesis and catalysis”, Alasdair I. McKay, James Barwick-Silk, Max Savage, Michael C. Willis and Andrew S. Weller,* Chem. Eur. J. 2020, 26, 2883-2889. (doi: 10.1002/chem.201904668).